(Printer-friendly PDF version | 536 KB )
(Large Print PDF version | 513 KB)
(Versión en español)
A number of research publications and federal reports document physical access barriers involving medical diagnostic equipment. This factsheet aims to inform health care providers about the laws and technical criteria that apply to accessible medical diagnostic equipment (MDE), in order to improve accessibility of MDE.
Affordable Care Act and Section 510 of the Rehabilitation Act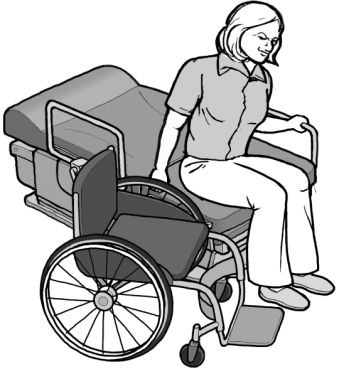
The “Patient Protection and Affordable Care Act” (ACA) added an amendment to Section 510 of the Rehabilitation Act which authorized the U.S. Access Board to develop accessibility standards for medical diagnostic equipment (MDE) in consultation with the Food and Drug Administration. The standards address independent access to, and use of, MDE by people with disabilities to the maximum extent possible. The standards for MDE apply to equipment that includes examination tables, examination chairs (including chairs used for eye examinations or procedures, and dental examinations or procedures), weight scales, mammography equipment, x-ray machines, and other radiological equipment commonly used for diagnostic purposes by health professionals.
Department of Justice, the ADA and Section 504 of the Rehabilitation Act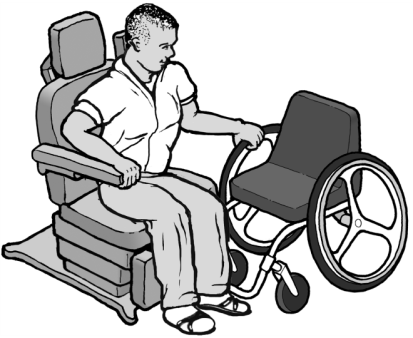
The Americans with Disabilities Act (ADA) and Section 504 of the Rehabilitation Act require health care providers to provide individuals with disabilities full and equal access to their health care services and facilities.
The Department of Justice and the Department of Health and Human Services recently published a guidance document for health care providers regarding their responsibilities to make their services and facilities accessible to individuals with mobility disabilities. See Access to Medical Care for Individuals with Mobility Disabilities in Resources. This guidance document includes information on accessible examination rooms and the clear floor space needed adjacent to medical equipment for individuals who use mobility devices to approach the equipment for transfer; accessible medical equipment (e.g., examination tables and chairs, mammography equipment, weight scales); patient lifts and other methods for transferring individuals from their mobility devices to medical equipment; and training health care personnel.
Standards for Accessible Medical Diagnostic Equipment (MDE Standards)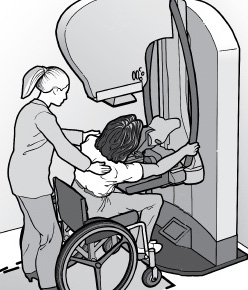
The MDE Standards, which became effective on February 8, 2017, establish minimum technical criteria that will allow patients with disabilities independent entry to, use of, and exit from medical diagnostic equipment to the maximum extent possible. For example, sections M301 and M302 of the MDE Standards address design and operational features that will allow a patient with a disability to transfer independently onto examination chairs and tables.
Note that the MDE Standards do not specify the minimum number of types of accessible medical equipment required in different types of health care facilities. In addition, these Standards are not yet enforceable as federal regulations. Refer to the Standards for Accessible Medical Diagnostic Equipment (see Resources).
Technical Criteria
Chapter M3 of the MDE Standards provides technical criteria for accessible diagnostic equipment based on the patient positions that the equipment is designed to support, including equipment used by patients: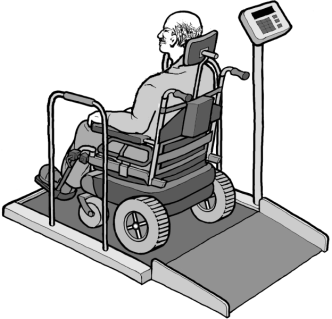
- in a supine, prone, or side-lying position (section M301); equipment used in a seated position (section M302)
- while seated in a wheelchair (section M303)
- in a standing position (section M304)
Chapter M3 also include technical criteria for equipment supports (section M305), for instructions or other information communicated to patients through the equipment (section M306), and for operable parts used by patients (section M307). Where equipment is designed to support more than one patient position, the equipment would have to meet the technical criteria for each position supported.
For more information on technical criteria of MDE Standards, see Resources below or call the ADA National Network at 1-800-949-4232.
Equipment Features Needed for Patient Support in Supine, Prone, or Side-Lying, Seated, Seated in Wheelchair or Standing Positions
|
Patient Positions Equipment Designed to Support |
Equipment Features Addressed by the Technical Criteria |
Examples of Types of Equipment that Apply to Patient Position |
|
M301 - Diagnostic Equipment Used by Patients in Supine, Prone, or Side-Lying Position |
|
|
|
M302 - Diagnostic Equipment Used by Patients in a Seated Position |
|
|
|
M303 - Seated in a wheelchair |
|
|
|
M304 - Standing position |
|
|
Resources
- Access Board - Standards for Accessible Medical Diagnostic Equipment
- Access Board – Health Care
- Department of Justice - Access to Medical Care for Individuals With Mobility Disabilities
- Example Applications of MDE Standards
- The Barrier Free Healthcare Initiative
|
Content was developed by the Northwest ADA Center, and is based on professional consensus of ADA experts and the ADA National Network. |
|
|
|
The contents of this factsheet were developed under grants from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant numbers 90DP0095 and 90DP0086). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this factsheet do not necessarily represent the policy of NIDILRR, ACL, HHS, and you should not assume endorsement by the Federal Government. |
|
© Copyright 2017 ADA National Network. All Rights Reserved. |
|

.png)





